Precision Denervation Powered by Real-Time Nerve Sensing
Autonomix Illuminates the Nervous System with First-of-Its-Kind Technology
Precision Denervation Powered by Real-Time Nerve Sensing
Autonomix is developing the world’s first GPS for the nervous system, a first-in-class, breakthrough platform technology that introduces real-time, intra-procedural nerve mapping. For the first time ever, this innovation has the potential to create a new paradigm in therapeutic electrophysiology by enabling physicians to sense nerve activity and guide treatment precision, representing a groundbreaking advancement in the field of transvascular denervation.
Protected by exclusive rights to a rapidly growing global IP portfolio – including 18 patent families and more than 120 issued or pending applications worldwide – Autonomix is building a category-defining solution with the potential to unlock new treatments across cardiology, interventional pain management, pulmonology, gastroenterology, and more.
First-In-Class System
This novel, minimally invasive approach allows for internal access to key organs and nerves through access to the vascular system, or vascular highway. By delivering targeted ablation transvascularly through the vessel wall, Autonomix opens up the possibility of transformative denervation treatment (selectively disabling nerves) across a number of disease categories including hypertension, chronic pain, cardiology, pulmonology, and gastroenterology through a single, first-in-class platform technology.
Revolutionizing Denervation
Autonomix’s technology is designed to enable physicians to sense, treat and verify the nerve is effectively ablated with an unprecedented precision that has the potential to revolutionize the way denervation is performed today, bringing targeted, nerve-specific denervation to the forefront and expanding possibilities for transvascular therapies.
Enabling Real-Time Intra-Procedural Nerve Mapping
Autonomix’s state-of-the-art system uniquely enables real-time nerve sensing, ablation, and verification giving physicians, for the first time ever, the ability to detect nerve activity, precisely guide energy delivery, optimize ablation patterns, and confirm that nerve signaling has stopped immediately during the procedure.
First-In-Class Precision
Detects and differentiates small, distributed nerve networks using high-sensitivity signal capture and local processing
120+ Patents Issued or Pending
Backed by a robust and globally protected IP portfolio covering 120+ patents issued or pending
Multi-Indication Platform
Designed to expand across a wide spectrum of therapeutic areas with high unmet need
Real-Time Feedback
Identifies nerve activity in real-time to guide clinicians to deliver effective therapy and confirm successful ablation
Personalized Therapy
Facilitates tailored therapy by mapping nerve activity so treatment can be adjusted based on individual physiology
A Growing List of Studies and Clinical Results
Autonomix’s initial focus is on interventional chronic pain management associated with severe pancreatic cancer in a first-in-human proof-of-concept study (PoC 1) to validate the concept.
Following promising initial results from PoC 1, the study has expanded to a follow-on phase that will double the potential addressable market beyond pancreatic cancer pain. The follow-on phase (PoC 2) will assess additional visceral cancers that signal pain through the Celiac Plexus and earlier stage pancreatic cancers with moderate to severe pain. This phase will provide a concentrated focus on interventional cancer pain management applications like pancreatic, gall bladder, liver, and bile duct, with potential further expansion in oncology, gastroenterology, and other sectors.
Insights and results from these valuable studies will guide the design of future clinical studies, including planned U.S. trials.

Clinical Results
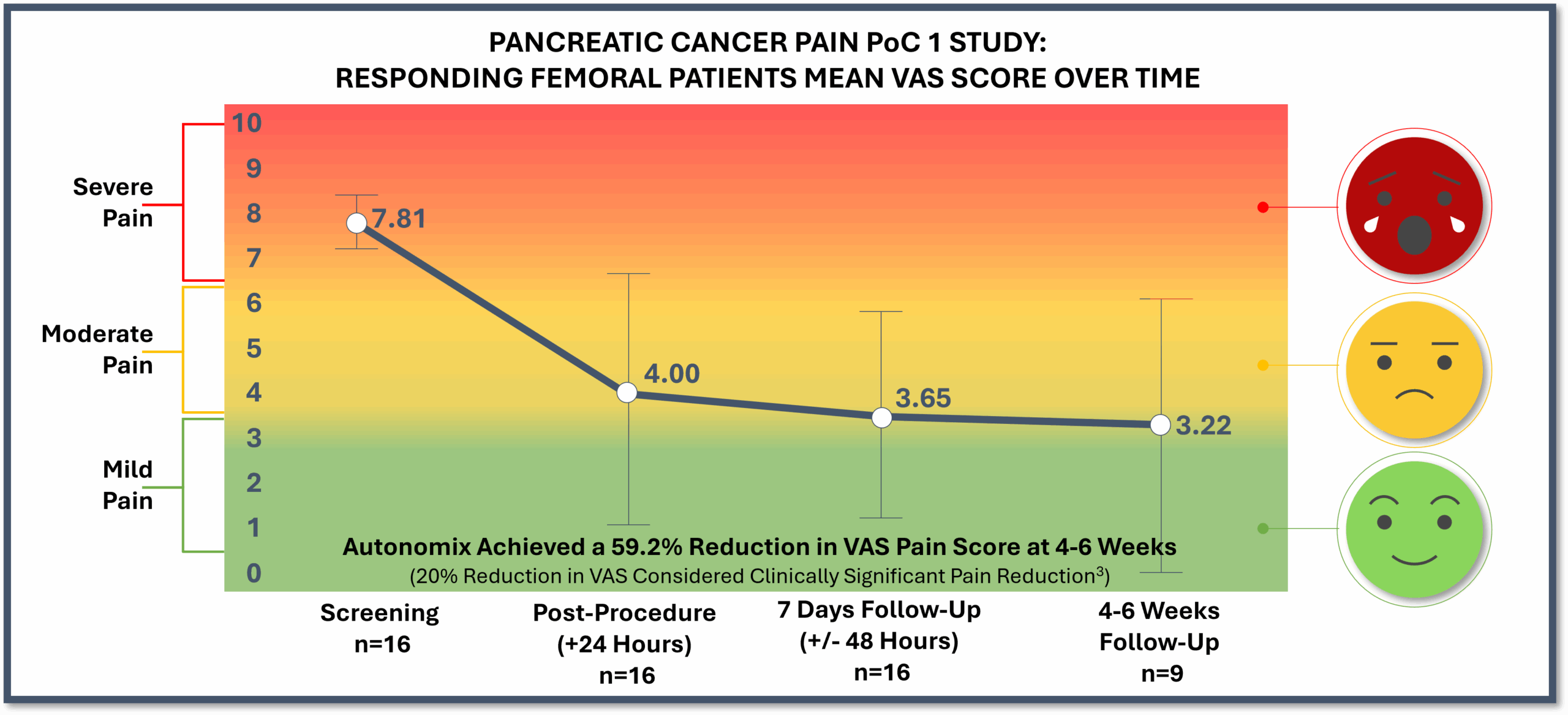
Results from PoC 1 Demonstrate Impact Across Critical Areas: Sustained Pain Reduction, 100% Zero Opioid Use, Quality of Life Gains, with No Device or Procedure-Related Serious Adverse Events
Strong Safety Profile
The procedure demonstrated a strong safety profile with no device or procedure-related serious adverse events1
Effective
Pain reduction remained consistently positive at 7-days and 4-6 weeks post-procedure
Rapid Relief
Statistically significant pain relief as early as 24-hours post procedure, providing patients with rapid relief
Meaningful Opioid Reduction
Responding patients were 100% opioid-free within a week of treatment, and 73% were still opioid-free 4-6 weeks post-procedure
1: As to be expected in seriously ill patient populations, there were 8 serious adverse events not related to the device or procedure (6 subjects succumbed to their disease before the 4-6 week follow up and 2 events related to disease progression resulting in hospitalization). In a separate clinical patient population of three subjects, there was a procedural-related serious adverse event of a hematoma at the access site resulting in hospitalization.
PoC 1 Topline Results Through 4-6 Week Post-Procedure Follow Up (N=20)
Study Design: One clinical site with 20 subjects1; Objective to transvascularly ablate relevant nerve plexuses to reduce intractable cancer pain
- Number of subjects includes the first five lead-in subjects used for procedure optimization.
- Three (3) patients with brachial access showed no improvement and one (1) patient was enrolled and not treated due to unsuccessful catheter placement because of an existing celiac trunk stenosis (narrowing of the vessel).
- Haefeli M, Elfering A. Pain assessment. Eur Spine J. 2006 Jan;15 Suppl 1(Suppl 1):S17-24. doi: 10.1007/s00586-005-1044-x. Epub 2005 Dec 1. PMID: 16320034; PMCID: PMC3454549.
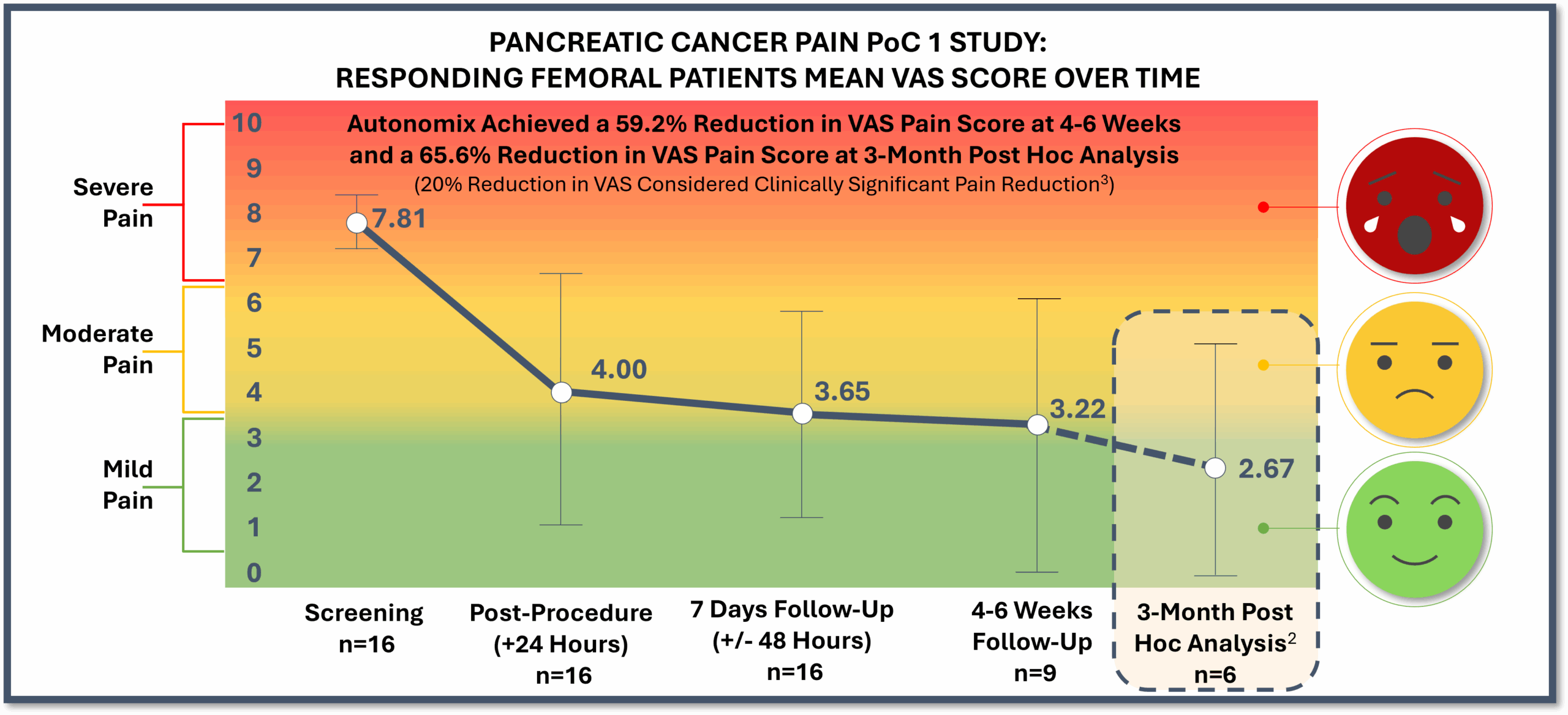
PoC 1 Post Hoc Analysis Results Through 3-Month1 Post-Procedure Follow Up (N=7)
Demonstrate sustained pain reduction, quality of life gains and 100% zero opioid use.
- Responder results (n=6)2 showed long-lasting, durable pain reduction of nearly 66%, or a mean 5.08 reduction on the VAS pain scale
- 100% of responders (n=7)2 showed zero opioid use
- Quality of life markers in responders (N=6)2 showed remarkable improvement in global health (mean 76.5% improvement), functional ability (mean 51.5% improvement), and symptom management (mean 50.4% improvement)
- As to be expected in seriously ill patient populations, the 3-month post-procedure follow up ranged from three to five months due to disease progression and the patient’s inability to travel.
- Of the 16 PoC 1 responding patients, a total of eight responders succumbed to their disease before the 3-month post-procedure follow-up which was attributed to disease progression and not related to the procedure. Two responding patient VAS and quality of life scores and one responding patient opioid status were not reported at the 3-month post-procedure follow-up due to the inability to travel given the natural progression of their disease and will be recorded as missing data in the final report.
- Haefeli M, Elfering A. Pain assessment. Eur Spine J. 2006 Jan;15 Suppl 1(Suppl 1):S17-24. doi: 10.1007/s00586-005-1044-x. Epub 2005 Dec 1. PMID: 16320034; PMCID: PMC3454549.
Autonomix’s technology is investigational and has not yet been cleared for marketing. This study was conducted using commercially available catheters (Cerablate Flutter® and RF Marinr™).
First-Hand Perspectives: Physicians & Patients
Hear from the primary investigator and participating patients about their experience in the PoC 1 study and the potential impact of this breakthrough technology.
Who We Help.
Advancing Innovation in Nervous System Technologies
Autonomix’s singular purpose is to transform patient outcomes by revolutionizing how diseases involving the peripheral nervous system are diagnosed and treated.
Initially, efforts are centered on pancreatic cancer patients suffering from intractable pain. Current therapies that primarily rely on opioids or invasive ethanol neurolysis can provide only limited relief and may lead to risky side effects.
Autonomix aims to deliver safe, effective, and lasting relief through a precision-guided, minimally invasive procedure to improve patient outcomes and quality of life. Once validated, the platform can be expanded to treat a wide array of conditions where overactive nerves play a critical role.
Robust and Globally-Protected IP Portfolio
Autonomix holds 120+ issued or pending patents spanning technologies, methods, and applications that underscore a breadth of application and expansion opportunities for Autonomix’s platform technology.
The Autonomix Opportunity
Proof-of-Concept in Lead Indication Unlocks a $100 Billion Market Potential
Autonomix is developing the world’s first GPS for the nervous system and approaching therapeutic electrophysiology in a new way by targeting shared vasculature and nerve plexuses involved in disease progression and symptom generation.
Platform Expansion Across Therapeutic Categories

Other Disorders May Also Include: Tumor Progression, Digestive Disorders, Urinary Tract Disorders, Metabolic Disorders and Chronic Inflammation.