Advancing Real-World Solutions for Patients Suffering from Intractable Nerve-Driven Pain
Clinical Trials Targeting
Pancreatic Cancer Pain
Advancing Real-World Solutions for Patients Suffering from Intractable Nerve-Driven Pain
Autonomix is redefining the treatment of pancreatic cancer pain through the development of a novel, minimally invasive nerve ablation platform. Our first-in-human clinical trial is focused on validating this technology in patients suffering from severe pancreatic cancer pain – a condition notoriously difficult to manage with existing therapies.
Autonomix’s lead clinical program has shown promising results in the initial phase (PoC 1) of our Proof-of-Concept study.
These early results offer important validation of our transvascular approach and reinforce the potential to address high-burden indications by enabling access to difficult-to-reach nerve bundles. Unlike traditional approaches that rely on open or percutaneous access, our catheter-based system allows for minimally invasive navigation through shared vasculature and reduces procedural complexity.
Results from PoC 1 Demonstrate Impact Across Critical Areas: Sustained Pain Reduction, 100% Zero Opioid Use, Quality of Life Gains, with No Device or Procedure-Related Serious Adverse Events
Strong Safety Profile
The procedure demonstrated a strong safety profile with no device or procedure-related serious adverse events1
Effective
Pain reduction remained consistently positive at 7-days and 4-6 weeks post-procedure
Rapid Relief
Statistically significant pain relief as early as 24-hours post procedure, providing patients with rapid relief
Meaningful Opioid Reduction
Responding patients were 100% opioid-free within a week of treatment, and 73% were still opioid-free 4-6 weeks post-procedure
- Of the 20 subjects enrolled, three (3) patients with brachial access showed no improvement and one (1) patient was enrolled and not treated due to unsuccessful catheter placement because of an existing celiac trunk stenosis (narrowing of the vessel).
- A total of four (4) responders succumbed to their disease before the 3-month post-procedure follow-up which was attributed to disease progression and not related to the procedure. Three (3) responding patient VAS scores, one (1) responding patient quality of life score and one (1) responding patient opioid status were not reported at the 4–6-week post-procedure follow-up due to the inability to travel given the natural progression of their disease and will be recorded as missing data in the final report.
- A total of eight (8) responders succumbed to their disease before the 3-month post-procedure follow-up which was attributed to disease progression and not related to the procedure. Two (2) responding patient VAS and quality of life scores and one (1) responding patient opioid status were not reported at the 3-month post-procedure follow-up due to the inability to travel given the natural progression of their disease and will be recorded as missing data in the final report.
PoC 1 Topline Results Through 4-6 Week Post-Procedure Follow Up (N=20)
Study Design: One clinical site with 20 subjects1; Objective to transvascularly ablate relevant nerve plexuses to reduce intractable cancer pain
- Number of subjects includes the first five lead-in subjects used for procedure optimization.
- Three (3) patients with brachial access showed no improvement and one (1) patient was enrolled and not treated due to unsuccessful catheter placement because of an existing celiac trunk stenosis (narrowing of the vessel).
- Haefeli M, Elfering A. Pain assessment. Eur Spine J. 2006 Jan;15 Suppl 1(Suppl 1):S17-24. doi: 10.1007/s00586-005-1044-x. Epub 2005 Dec 1. PMID: 16320034; PMCID: PMC3454549.
Read the Full Topline Results
- All patients entered the study with severe abdominal pain from unresectable pancreatic cancer and a life expectancy of 3 months or more
- 19 of 20 enrolled patients were treated, and each assessed as successful catheter placement in the celiac trunk. One (1) patient was enrolled and not treated due to unsuccessful catheter placement because of an existing celiac trunk stenosis (narrowing of the vessel)
- There were no device or procedure-related serious adverse events. As to be expected with surgical procedures in seriously ill patient populations, there were 8 serious adverse events (6 subjects succumbed to their disease before the 4-6 week follow up, which were not related to the procedure, and 2 events resulting in hospitalization also unrelated to the procedure) and 14 adverse events (including 8 events of expected arterial constrictions due to spasms and temporary artery occlusion)
- 16 patients were treated using femoral access and three (3) patients using brachial access. 100% of patients (16) with femoral access responded to treatment, while the three (3) patients with brachial access showed no improvement in pain scores, representing a key procedural learning
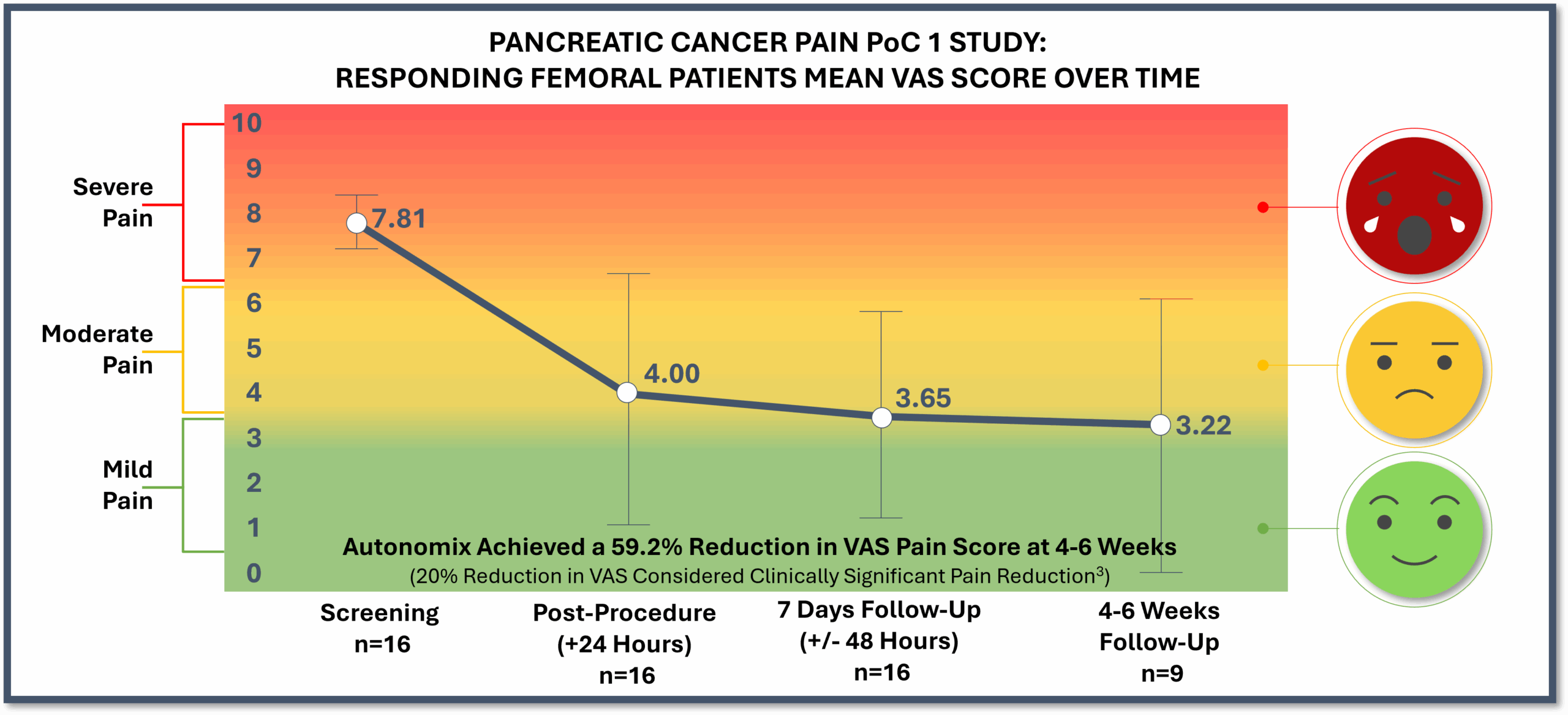
- Across the total population (mITT – n=19), pain relief occurred as early as 24 hours post-procedure. At 7-days post-procedure, there was a mean pain reduction of 3.32 on the Visual Analog Scale (“VAS”) (baseline 7.61 to 4.29), or 43.6% improvement. At 4-6 weeks post-procedure, there was a mean pain reduction of 3.95 on the VAS pain scale (baseline of 7.95 to 4.00), or 49.7% improvement
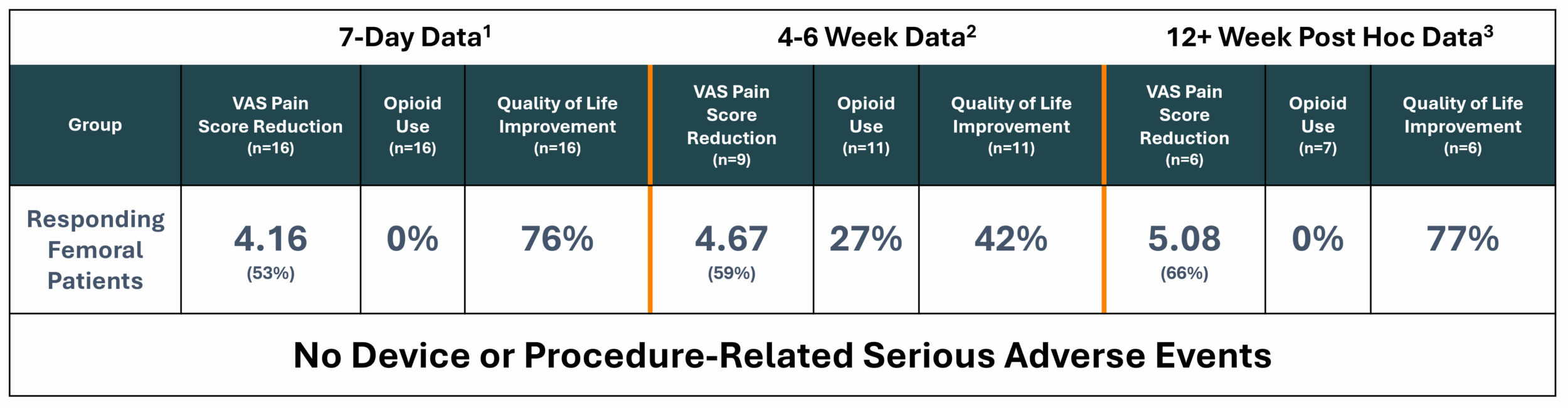
- Responding femoral patients (n=16) represented 84% of treated patients with a mean pain reduction of 4.16 on the VAS pain scale (baseline of 7.81 to 3.65), or 53.3% improvement, at 7-days post-procedure. At 4-6 weeks post-procedure, there was a mean 4.67 reduction on the VAS pain scale (baseline of 7.89 to 3.22), or 59.2% improvement
- At 7-days post-procedure, responding femoral patients reported a 76% improvement in global quality of health, a 33% improvement in functional quality of life and a 37% improvement in symptomatic quality of life. At 4-6 weeks post-procedure, responding femoral patients reported a 42% improvement in global quality of health, a 28% improvement in functional quality of life and a 29% improvement in symptomatic quality of life
- 100% of responding patients were able to go to zero opioid use at 7-days post-procedure, while 73% of responding patients were at zero opioid use at 4-6 weeks post-procedure
- Three responding patient VAS scores, one responding patient quality of life score and one responding patient opioid status were not reported at the 4–6-week post-procedure follow-up due to the inability to travel given the natural progression of their disease and will be recorded as missing data in the final report
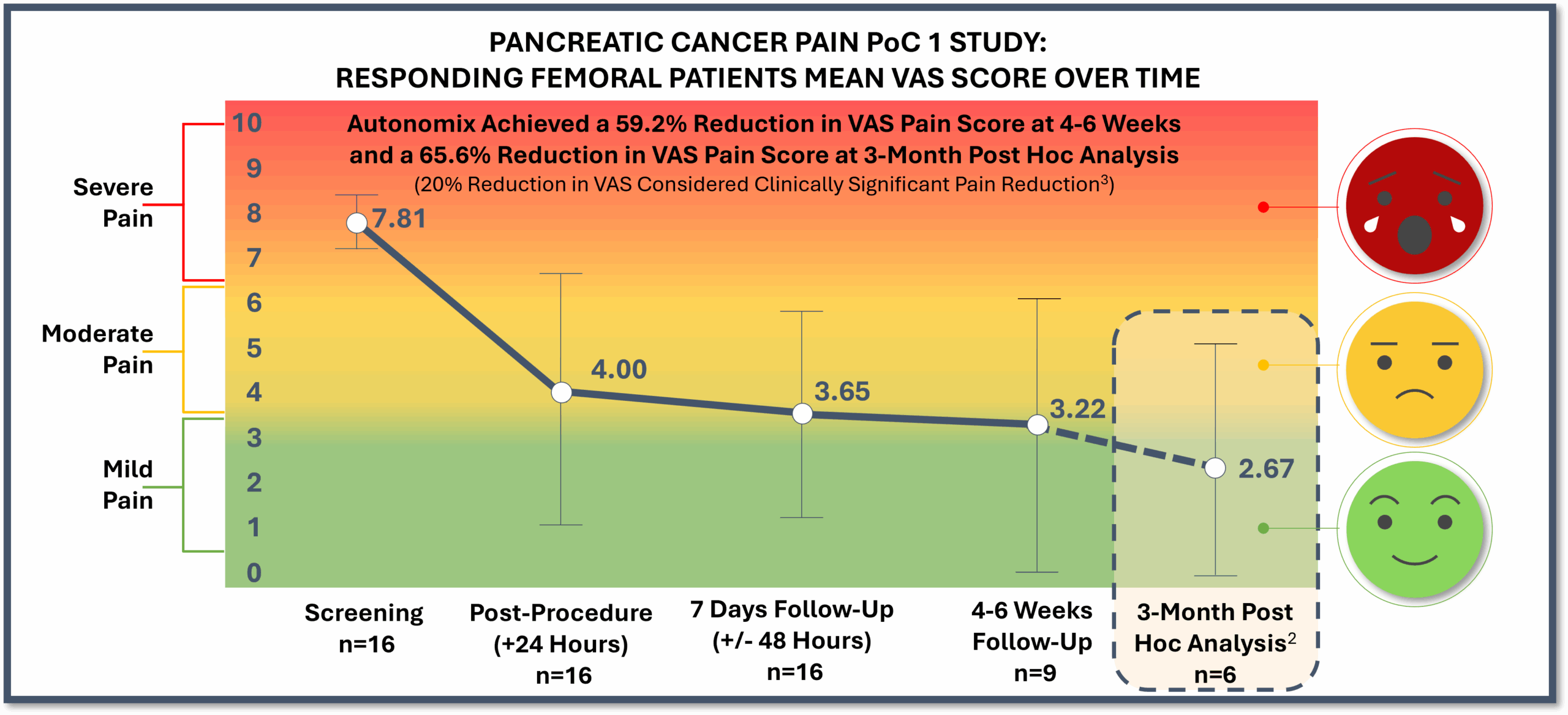
PoC 1 Post Hoc Analysis Results Through 3-Month1 Post-Procedure Follow Up (N=7)
Demonstrate sustained pain reduction, quality of life gains and 100% zero opioid use.
- Responder results (n=6)2 showed long-lasting, durable pain reduction of nearly 66%, or a mean 5.08 reduction on the VAS pain scale
- 100% of responders (n=7)2 showed zero opioid use
- Quality of life markers in responders (N=6)2 showed remarkable improvement in global health (mean 76.5% improvement), functional ability (mean 51.5% improvement), and symptom management (mean 50.4% improvement)
- As to be expected in seriously ill patient populations, the 3-month post-procedure follow up ranged from three to five months due to disease progression and the patient’s inability of the patient to travel.
- Of the 16 PoC 1 responding patients, a total of eight responders succumbed to their disease before the 3-month post-procedure follow-up which was attributed to disease progression and not related to the procedure. Two responding patient VAS and quality of life scores and one responding patient opioid status were not reported at the 3-month post-procedure follow-up due to the inability to travel given the natural progression of their disease and will be recorded as missing data in the final report.
- Haefeli M, Elfering A. Pain assessment. Eur Spine J. 2006 Jan;15 Suppl 1(Suppl 1):S17-24. doi: 10.1007/s00586-005-1044-x. Epub 2005 Dec 1. PMID: 16320034; PMCID: PMC3454549.
Read the Full Topline Results
- Responding patients (n=6)1 reported a mean 5.08 pain reduction on the VAS pain scale (baseline of 7.75 to 2.67), or 65.6% improvement.
- Among responders (N=7)1, 100% of patients were opioid free at their 3-month post-procedure follow-up which highlights the potential for a non-opioid alternative in a highly opioid-reliant disease.
- Responding patients (N=6)1 experienced remarkable improvement in global health (mean 76.5% improvement), functional ability (mean 51.5% improvement), and symptom management (mean 50.4% improvement), demonstrating the procedure addresses more than just pain and has the potential to improve day-to-day living during end-stage cancer.
- Specific quality of life markers further improved including sleep quality, energy level and ability to lead normal daily and leisure time activities and reduced tension, vomiting and constipation.
Autonomix’s technology is investigational and has not yet been cleared for marketing. This study was conducted using commercially available catheters (Cerablate Flutter® and RF Marinr™).
Physician and Lead-In Patient Interviews from Initial Phase of Proof-of-Concept Study
Hear from the primary investigator and participating patients about their experience in the PoC trial and the potential impact of this breakthrough technology.
Proof-of-Concept Expansion (PoC 2): From Severe Pancreatic Cancer Pain to Broader Visceral Applications
Targeting pain in additional visceral cancers and earlier-stage pancreatic cancer
Building on the positive results demonstrated in the PoC 1 phase, Autonomix will initiate a follow-on PoC 2 phase in a market expansion opportunity that will double the potential addressable market beyond pancreatic cancer pain by evaluating additional visceral cancers that signal pain through the Celiac Plexus and earlier stage pancreatic cancers with moderate to severe pain. This follow-on phase of development is designed to validate the broader utility of our technology in interventional cancer pain management.
Study Design
20
Subjects
01
Clinical Site
Objective
Transvascular Ablation of Relevant Nerve Plexuses to Reduce Intractable Cancer Pain
- Visceral Cancer Pain Management: Focus on Interventional Cancer Pain Management in Visceral Cancers, including: Pancreatic | Gall Bladder | Liver | Bile Duct
- Earlier Stage Pancreatic Cancer: Expanding Treatment to Patients with Moderate to Severe Pain, Earlier in Disease Progression
- Market Impact: This Expansion Has the Potential to Double the Addressable Market Beyond Late-Stage Pancreatic Cancer Pain